INTRODUCTION: When two monosaccharides are combined together by glycosidic linkage, a disaccharide is formed. The important disaccharides are; 1) Sucrose 2) Maltose and isomaltose 3) Lactose.
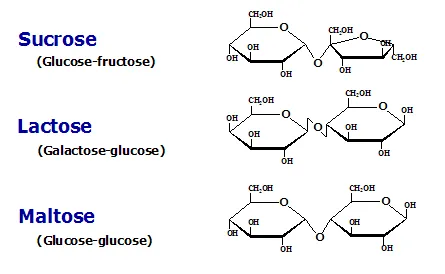
SUCROSE: It is the sweetening agent known as cane sugar, present in sugarcane and various fruits. Sucrose contains glucose and fructose. Sucrose is not a reducing sugar; and it will not form osazone. This is because the linkage involves first carbon of glucose and second carbon of fructose, and free reducing groups are not available. When sucrose is hydrolysed, the products have reducing action. During laboratory investigations, a sugar solution which is originally non-reducing then becomes reducing after hydrolysis, and is identified as sucrose. This serves as the specific sucrose test.
LACTOSE: It is the sugar present in milk. It is a reducing disaccharide. On hydrolysis lactose yields glucose and galactose. Beta glycosidic linkage is present in lactose. The anomeric carbon atom of beta-galactose is attached to the 4th hydroxyl group of glucose through beta-1,4 glycosidic linkage. The lactose may be alpha or beta variety, depending on the configuration of 1st carbon of glucose moiety.
MALTOSE: Maltose contains two glucose residues. There is alpha-1,4 linkage, that is to say, the anomeric 1st carbon atom of one glucose is combined with 4th hydroxyl group of another glucose through alpha-glycosidic linkage. Maltose may be alpha or beta depending on the configuration at the free anomeric carbon atom. It is a reducing disaccharide. Isomaltose It is also a reducing sugar. It contains 2 glucose units combined in alpha -1, 6 linkage. Thus first carbon of one glucose residue is attached to the sixth carbon of another glucose through a glycosidic linkage. Partial hydrolysis of glycogen and starch produces isomaltose. The enzyme oligo1,6-glucosidase present in intestinal juice can hydrolyse isomaltose into glucose units.
RELATED;













No comments:
Post a Comment