INTRODUCTION: Fibrinolytic drugs rapidly lyse thrombi by catalyzing the formation of the serine protease plasmin from its precursor zymogen, plasminogen. These drugs create a generalized lytic state when administered intravenously. Thus, both protective hemostatic thrombi and target thromboemboli are broken down. Myocardial Infarction describes the use of these drugs in one major application.
PHARMACOLOGY: Streptokinase is a protein (but not an enzyme in itself) synthesized by streptococci that combines with the proactivator plasminogen. Streptococci
This enzymatic complex catalyzes the conversion of inactive plasminogen to active plasmin.
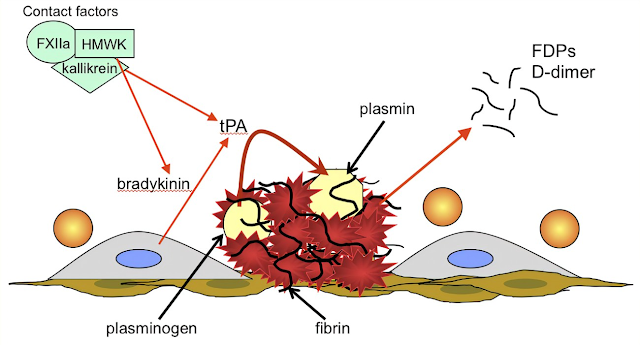
Urokinase is a human enzyme synthesized by the kidney that directly converts plasminogen to active plasmin. Plasmin itself cannot be used because naturally occurring inhibitors in plasma prevent its effects. However, the absence of inhibitors for urokinase and the streptokinase-proactivator complex permits their use clinically. Plasmin formed inside a thrombus by these activators is protected from plasma antiplasmins, which allows it to lyse the thrombus from within.
Anistreplase (anisoylated plasminogen streptokinase activator complex; APSAC) consists of a complex of purified human plasminogen and bacterial streptokinase that has been acylated to protect the enzyme’s active site. When administered, the acyl group spontaneously hydrolyzes, freeing the activated streptokinase-proactivator complex. This product allows for rapid intravenous injection, greater clot selectivity (ie, more activity on plasminogen associated with clots than on free plasminogen in the blood), and more thrombolytic activity. Plasminogen can also be activated endogenously by tissue plasminogen activators (t-PAs). These activators preferentially activate plasminogen that is bound to fibrin, which confines fibrinolysis to the formed thrombus and avoids systemic activation. Human t-PA is manufactured as alteplase by means of recombinant DNA technology.
Reteplase is another recombinant human t-PA from which several amino acid sequences have been deleted. Reteplase is less expensive to produce than t-PA. Because it lacks the major fibrin-binding domain, reteplase is less fibrinspecific than t-PA.
Tenecteplase is a mutant form of t-PA that has a longer half-life, and it can be given as an intravenous bolus. Tenecteplase is slightly more fibrin-specific than t-PA.
INDICATIONS & DOSAGE: Administration of fibrinolytic drugs by the intravenous route is indicated in cases of pulmonary embolism with hemodynamic instability, severe deep venous thrombosis such as the superior vena caval syndrome, and ascending thrombophlebitis of the iliofemoral vein with severe lower extremity edema. Thromboticdisorders
These drugs are also given intra-arterially, especially for peripheral vascular disease. Thrombolytic therapy in the management of acute myocardial infarction requires careful patient selection, the use of a specific thrombolytic agent, and the benefit of adjuvant therapy. Streptokinase is administered by intravenous infusion of a loading dose of 250,000 units, followed by 100,000 units/h for 24–72 hours. Patients with antistreptococcal antibodies can develop fever, allergic reactions, and therapeutic resistance. Drug resistance
Urokinase requires a loading dose of 300,000 units given over 10 minutes and a maintenance dose of 300,000 units/h for 12 hours. Alteplase (t-PA) is given by intravenous infusion of 60 mg over the first hour and then 40 mg at a rate of 20 mg/h. Reteplase is given as two intravenous bolus injections of 10 units each, separated by 30 minutes. Tenecteplase is given as a single intravenous bolus of 0.5 mg/kg. Anistreplase (where available) is given as a single intravenous injection of 30 units over 3–5 minutes. Recombinant t-PA has also been approved for use in acute ischemic stroke within 3 hours of symptom onset. In patients without hemorrhagic infarct or other contraindications, this therapy has been demonstrated to provide better outcomes in several randomized clinical trials. Clinicaltrials
The recommended dose is 0.9 mg/kg, not to exceed 90 mg, with 10% given as a bolus and the remainder during a 1 hour infusion. Streptokinase has been associated with increased bleeding risk in acute ischemic stroke when given at a dose of 1.5 million units, and its use is not recommended in this setting.
RELATED;











No comments:
Post a Comment